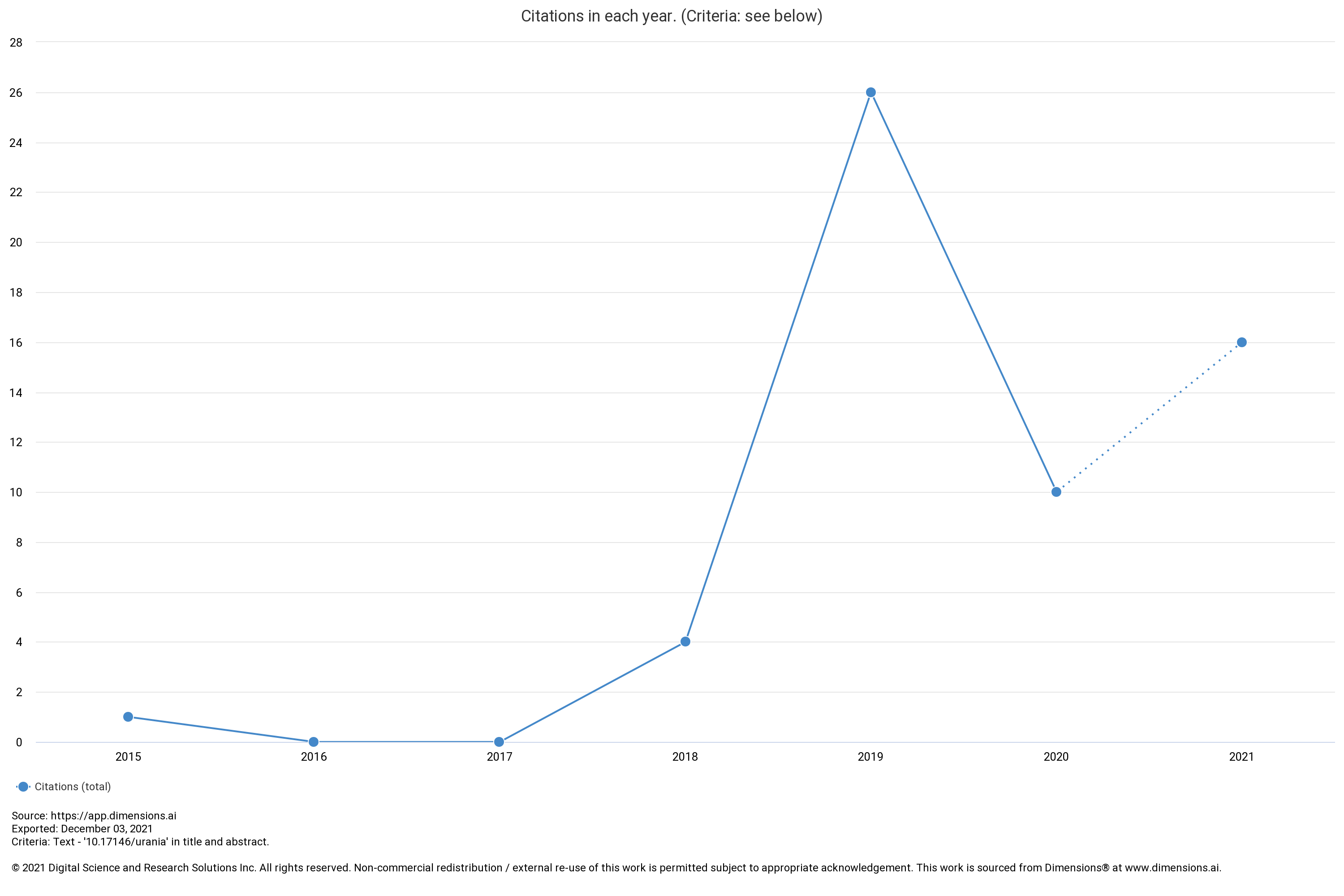
Effect Of Current, Time, Feed and Cathode Type On Electroplating Process Of Uranium Solution
DOI: http://dx.doi.org/10.17146/urania.2017.23.1.3155
Sari
Electroplating process of uranyl nitrate and effluent process has been carried out in order to collect uranium contained therein using electrode Pt / Pt and Pt / SS at various currents and times. Material used for electrode were Pt (platinum) and SS (Stainlees Steel). Feed solution of 250 mL was entered into a beaker glass equipped with Pt anode - Pt cathode or Pt anode - SS cathode, then fogged direct current from DC power supply with specific current and time so that precipitation of uranium sticking to the cathode. After the processes completed, the cathode was removed and weighed to determine weight of precipitates, while the solution was analyzed to determine the uranium concentration decreasing after and before electroplating process. The experiments showed that a relatively good time to acquire uranium deposits at the cathode was 1 hour by current 7 ampere, uranyl nitrate as feed, and Pt (platinum) as cathode. In these conditions, uranium deposits attached to the cathode amounted to 74.96% of the original weight of uranium oxide in the feed or 206.5 mg weight. The use of Pt cathode for uranyl nitrate, SS and Pt cathode for effluent process feed gave uranium specific weight at the cathode of 12.99 mg/cm2, 2.4 mg/cm2 and 5.37 mg/cm2respectively for current 7 ampere and electroplating time 1 hour.
Keywords: Electroplating, uranyl nitrate, effluent process, Pt/Pt electrode, Pt/SS electrode
Teks Lengkap:
PDFRefbacks
- Saat ini tidak ada refbacks.
Penerbit: Pusat Riset Teknologi Bahan Nuklir dan Limbah Radioaktif
Diindeks oleh: